Today, I am presenting a case study of what happens when a Covered Entity (CE) and a pharmaceutical company collude to violate the Federal Anti-Kickback Statute and the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule.
Healthcare has a Federal Anti-kickback Statute (AKS), 42 U.S.C. § 1320a-7b(b), that makes it illegal for providers to knowingly and willfully accept bribes or other forms of remuneration in return for generating Medicare, Medicaid or ANY other federal health care program business.
What does remuneration mean under the Anti-Kickback Statute?
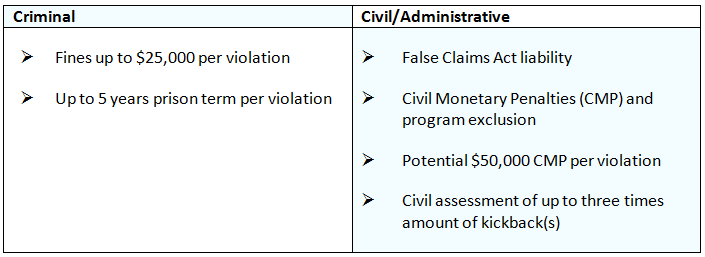
The AKS provides criminal penalties, as defined in the table below, for individuals and entities that knowingly and willfully offer, pay, solicit or receive remuneration in order to induce business(es) for which payment(s) may be made under a federal healthcare program.
Kickbacks in healthcare have lead to:
- → Over utilization
- → Increased program costs
- → Corruption of medical decision making
- → Patient steering
- → Unfair competition
Did You Know?
The kickback prohibition applies to ALL sources of referrals, even patients. For example, where the Medicare and Medicaid programs require patients to pay co-pays for services and CEs are generally required to collect that money from your patients.
Routinely waiving co-pays could activate the AKS and you are not allowed to advertise that you will forgive co-payments. However, you are free to waive a co-payment IF you make an individual determination that the patient cannot afford to pay or if your reasonable collection efforts fail.
The Government does not need to prove patient harm or financial loss to the programs to show that a physician violated the AKS. A physician can be guilty of violating the AKS even if the physician actually rendered the service and the service was medically necessary.
Taking money or gifts from a drug or device company or a durable medical equipment (DME) supplier is not justified by the argument that you would have prescribed that drug or ordered that wheelchair even without a kickback.
Now that I’ve shared all that with you it is time for… Drum Roll Please!
Case Study: What Happens When Drug Company Offers Kick-backs To Doctors
It doesn’t happen often, but when it does, the Department of Justice (DOJ) WILL impose criminal penalties for ANY HIPAA violation(s).
This is one such case that resulted in two criminal convictions – a violation of HIPAA and obstructing a criminal healthcare investigation.
Here is what happened:
From January 2011 through November 2011, a Massachusetts gynecologist allowed a pharmaceutical company sales representative from Warner Chilcott to access the protected health information (PHI) in her patients’ medical files. When questioned later, she later provided false information to federal agents when interviewed about her relationship with Warner Chilcott.
On October 29, 2015, Warner Chilcott U.S. Sales LLC, a subsidiary of pharmaceutical manufacturer Warner Chilcott PLC, pled guilty to paying kickbacks to induce physicians to prescribe their drugs.
Warner Chilcott agreed to pay $125 Million to resolve criminal liability and several False Claims Acts allegations.
The DOJ investigation didn’t end there; In September 2018, the gynecologist was sentenced to one year of probation for violating HIPAA and one count of obstruction of a criminal healthcare investigation.
Covered Entities and Business Associates need to understand your patients are entrusting YOU with their most private and intimate details, they expect it to remain secure.
Besides, it is YOUR practice, YOUR patients, YOUR reputation, and YOUR legacy! Why are you leaving yourself wide open to such risks?

For tips like this and more request your copy of our “HIPAA Security Rule – Know The Rules!” Newsletter Today.
